NIH Definitions
NIMICT Original
NIH’S Definitions
Quick definitions to understand the NIH Policy
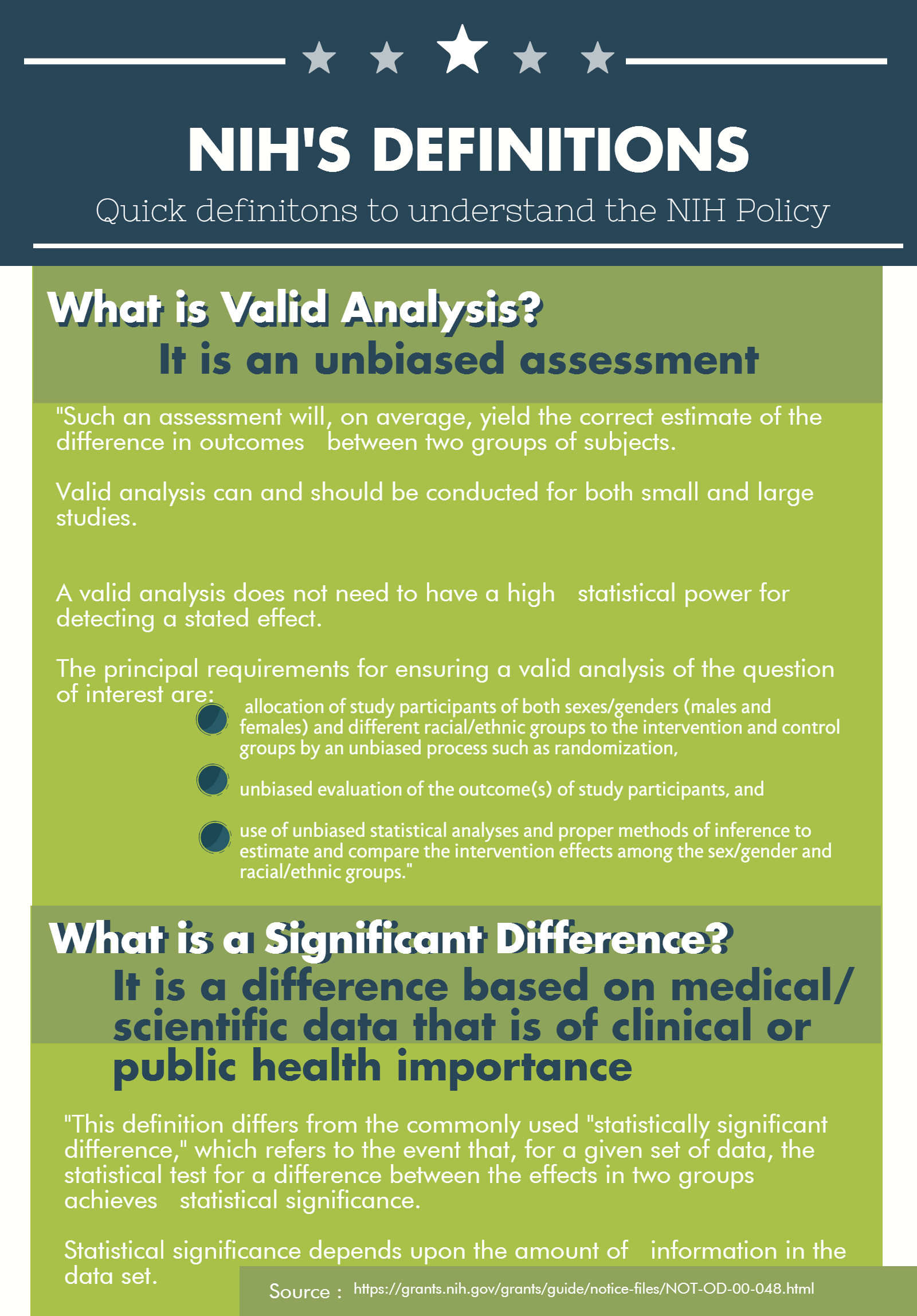
What is Valid Analysis?
It is an unbiased assessment
- "Such an assessment will, on average, yield the correct estimate of the difference in outcomes between two groups of subjects.
- Valid analysis can and should be conducted for both small and large studies.
- A valid analysis does not need to have a high statistical power for detecting a stated effect.
- The principal requirements for ensuring a valid analysis of the question of interest are:
- allocation of study participants of both sexes/genders (males and females) and different racial/ethnic groups to the intervention and control groups by an unbiased process such as randomization,
- unbiased evaluation of the outcome(s) of study participants, and
- use of unbiased statistical analyses and proper methods of inference to estimate and compare the intervention effects among the sex/gender and racial/ethnic groups."
What is a Significant Difference?
It is a difference based on medical/ scientific data that is of clinical or public health importance
- "This definition differs from the commonly used "statistically significant difference," which refers to the event that, for a given set of data, the statistical test for a difference between the effects in two groups achieves statistical significance.
- Statistical significance depends upon the amount of information in the data set.
Other NIMICT Tools