Coverage of Clinical Trials
NIMICT Orginal
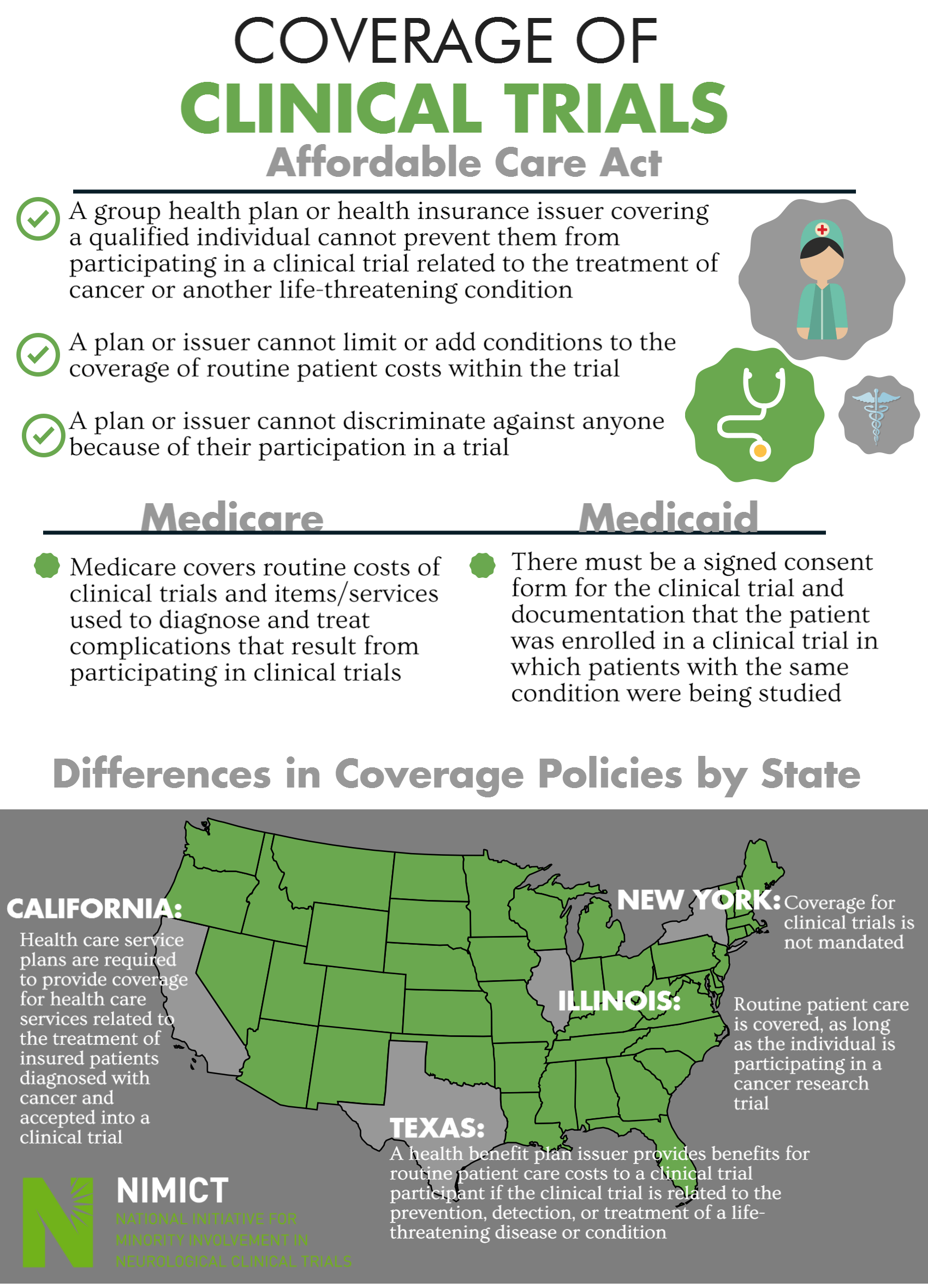
Coverage of Clinical Trials
Affordable Care Act
- A group health plan or health insurance issuer covering a qualified individual cannot prevent them from participating in a clinical trial related to the treatment of cancer or another life-threatening condition
- A plan or issuer cannot limit or add conditions to the coverage of routine patient costs within the trial
- A plan or issuer cannot discriminate against anyone because of their participation in a trial
Medicare
- Medicare covers routine costs of clinical trials and items/services used to diagnose and treat complications that result from participating in clinical trials
- There must be a signed consent form for the clinical trial and documentation that the patient was enrolled in a clinical trial in which patients with the same condition were being studied
Differences in Coverage Policies by State
- California
- Health care service plans are required to provide coverage for health care services related to the treatment of insured patients diagnosed with cancer and accepted into a clinical trial
- Texas
- A health benefit plan issuer provides benefits for routine patient care costs to a clinical trial participant if the clinical trial is related to the prevention, detection, or treatment of a life-threatening disease or condition
- Illinois
- Routine patient care is covered, as long as the individual is participating in a cancer research trial
- New York
- Coverage for clinical trials is not mandated
Other NIMICT Tools