Rules and Regulation Overview
NIMICT Original
Human Subjects Research
Regulations, Rules, and Research Oversight
Knowing the requirements of your funding source dictates the protocol and regulations.
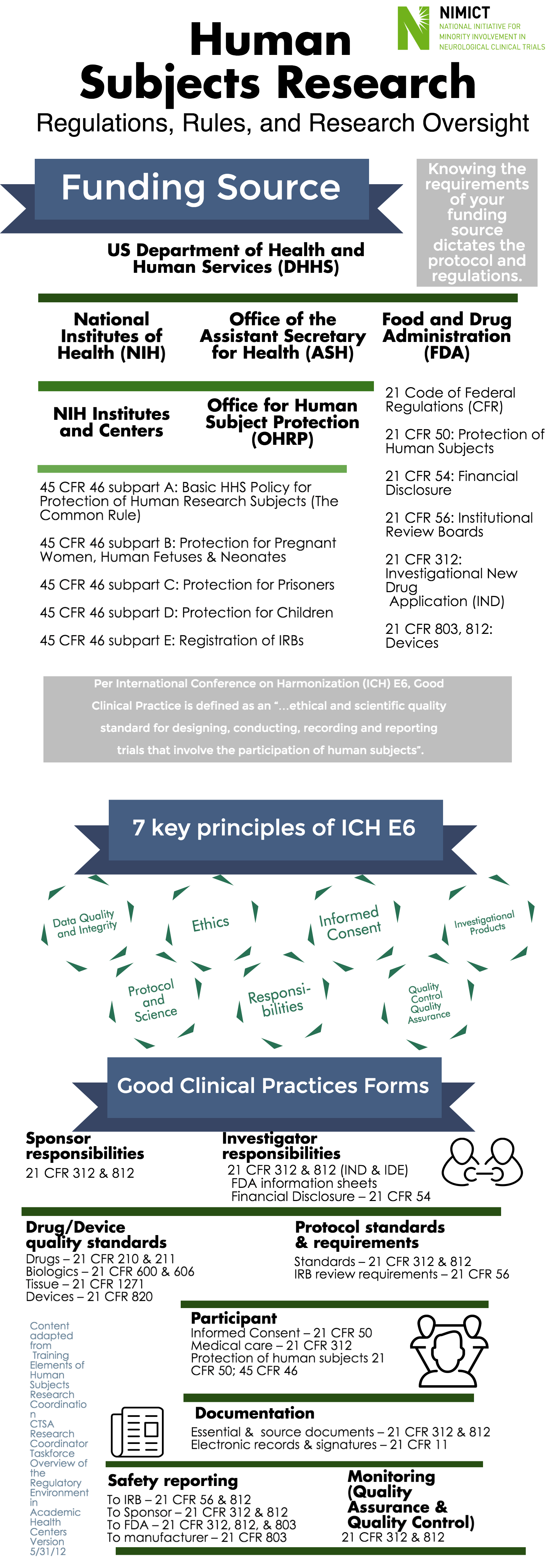
Funding Source
US Department of Health and Human Services (DHHS), National Institutes of Health (NIH) Office of the Assistant Secretary for Health (ASH), NIH Institutes and Centers, Office for Human Subject Protection (OHRP)
-45 CFR 46 subpart A: Basic HHS Policy for Protection of Human Research Subjects (The Common Rule)
-45 CFR 46 subpart B: Protection for Pregnant Women, Human Fetuses & Neonates
-45 CFR 46 subpart C: Protection for Prisoners
-45 CFR 46 subpart D: Protection for Children
-45 CFR 46 subpart E: Registration of IRBs
Food and Drug Administration (FDA)
-21 Code of Federal Regulations (CFR)
-21 CFR 50: Protection of Human Subjects
-21 CFR 54: Financial Disclosure
-21 CFR 56: Institutional Review Boards
-21 CFR 312: Investigational New Drug Application (IND)
-21 CFR 803, 812: Devices
Per International Conference on Harmonization (ICH) E6, Good Clinical Practice is defined as an “…ethical and scientific quality standard for designing, conducting, recording and reporting trials that involve the participation of human subjects”.
7 key principles of ICH E6
-Data Quality and Integrity
-Ethics
-Investigational Products
-Protocol and Science
-Responsibilities
-Quality Control Quality Assurance
Good Clinical Practices Forms
Sponsor responsibilities
21 CFR 312 & 812
Investigator responsibilities
21 CFR 312 & 812 (IND & IDE)
FDA information sheets
Financial Disclosure – 21 CFR 54
Drug/Device quality standards
Drugs – 21 CFR 210 & 211
Biologics – 21 CFR 600 & 606
Tissue – 21 CFR 1271
Devices – 21 CFR 820
Protocol standards & requirements
Standards – 21 CFR 312 & 812
IRB review requirements – 21 CFR 56
Informed Consent – 21 CFR 50
Medical care – 21 CFR 312
Protection of human subjects 21
CFR 50; 45 CFR 46
Documentation
Essential & source documents – 21 CFR 312 & 812
Electronic records & signatures – 21 CFR 11
Safety reporting
To IRB – 21 CFR 56 & 812
To Sponsor – 21 CFR 312 & 812
To FDA – 21 CFR 312, 812, & 803
To manufacturer – 21 CFR 803
Monitoring (Quality Assurance & Quality Control)
21 CFR 312 & 812
*Content adapted from Training Elements of Human Subjects Research Coordination CTSA Research Coordinator Taskforce Overview of the Regulatory Environment in Academic Health Centers Version 5/31/12