Coordinator Responsibilities
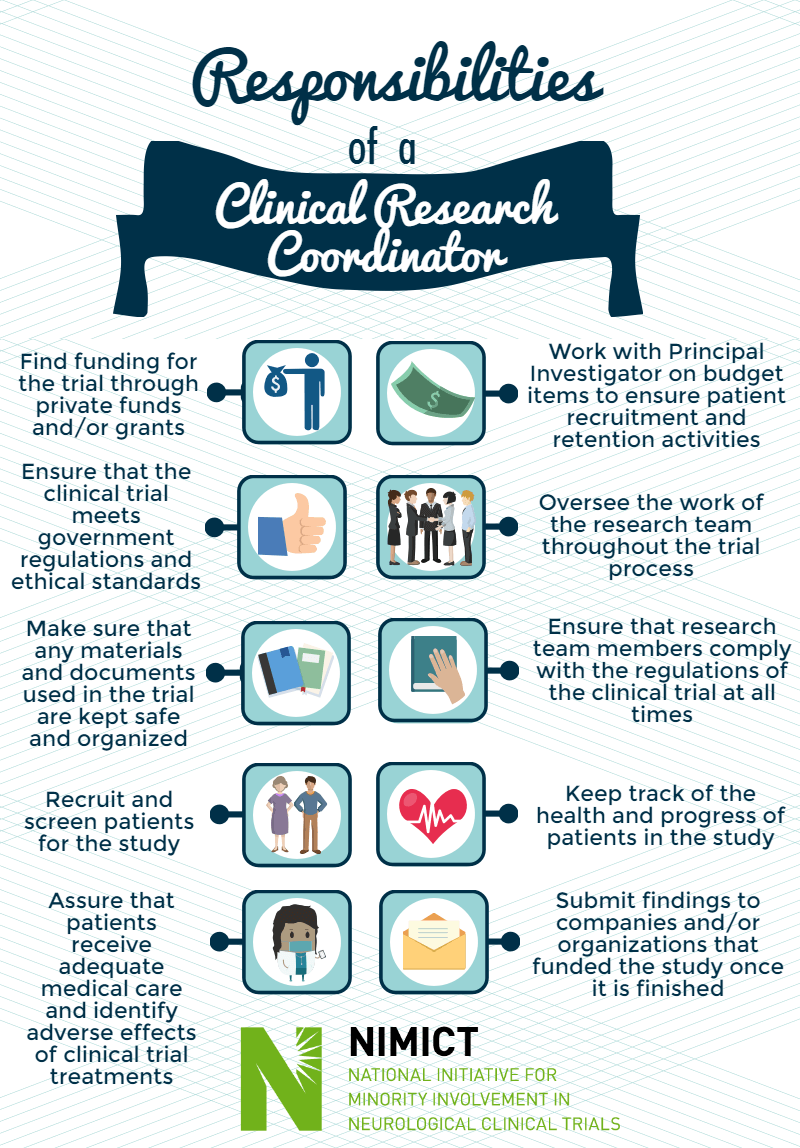
Responsibilities of a Clinical Research Coordinator
- Find funding for the trial through private funds and/or grants
- Work with Principal Investigator on budget items to ensure patient recruitment and retention activities
- Ensure that the clinical trial meets government regulations and ethical standards
- Oversee the work of the research team throughout the trial process
- Make sure that any materials and documents used in the trial are kept safe and organized
- Ensure that research team members comply with the regulations of the clinical trial at all times
- Recruit and screen patients for the study
- Keep track of the health and progress of patients in the study
- Assure that patients receive adequate medical care and identify adverse effects of clinical trial treatments
- Submit findings to companies and/or organizations that funded the study once it is finished
Infographic