3 Simple Rules
Three simple rules to ensure reasonably credible subgroup analyses
This resource outlines rules of thumb for determining when subgroup analyses can be performed as hypothesis testing analyses. Download the case study or click here to view full text.
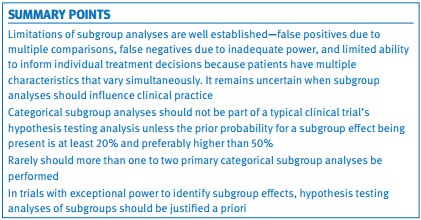
Summary points
-
Limitations of subgroup analyses are well established—false positives due to multiple comparisons, false negatives due to inadequate power, and limited ability to inform individual treatment decisions because patients have multiple characteristics that vary simultaneously. It remains uncertain when subgroup analyses should influence clinical practice
-
Categorical subgroup analyses should not be part of a typical clinical trial’s hypothesis testing analysis unless the prior probability for a subgroup effect being present is at least 20% and preferably higher than 50%
-
Rarely should more than one to two primary categorical subgroup analyses be performed
-
In trials with exceptional power to identify subgroup effects, hypothesis testing analyses of subgroups should be justified a priori